It is the responsibility of a top quality department to depict the organization all through regulatory inspections. So, For that reason, the quality Division should approve the final CAPA procedure draft.
Corrective Action and Preventive action are the basic top quality administration equipment which embrace quite a few methods taken to eliminate, right or take care of the defect or undesirable circumstance. They give full attention to constant improvement and varieties an indispensable part of any enterprise.
Accredited programs for people and privacy professionals who want the very best-excellent instruction and certification.
On the flip side, Preventive Action involves carrying out pattern Evaluation to discover the problems which may lead to non-conformity and address them to be able to stay away from nonconformity.
A CAPA report and CAPA kind are fundamentally the identical matters. When staff fills the CAPA form Using the suitable info and subsequent acceptance by approved staff, the CAPA sort’s status improvements for the CAPA report.
Connect which has a MasterControl representative these days to find how our industry-foremost CAPA solution streamlines high-quality administration.
CAPA is among the very best vital excellent programs based on the FDA. Corrective action and preventive action can be used independently or be applied jointly.
Incidents: An incident refers to an unexpected or unplanned occasion that deviates from standard functions, procedures, or anticipations within a corporation
Status updates through the Efficiency Monitoring phase shall be designed quarterly, at a minimum amount, If your target effectiveness checking completion date is bigger than ninety (ninety) days.
What are a few Frequent Troubles with CAPA in the Pharmaceutical Industry? A number of the prevalent challenges pharma organizations deal with in utilizing continuous action preventive action contain Guide procedures that hinder the identification of probable risks and issues in solution and excellent until it is simply too late. If the foundation Bring about Assessment is effective, it could leave the corrective and preventive action difference organization a lot more vulnerable as the condition proceeds to occur or requires new types.
Our connected suite of alternatives helps firms of all dimensions improve merchandise, quality, security, and supplier as they convey their products from strategy to customer good results. Fulfill the Management Team
It can be important to produce a CAPA process that's understandable to all functions. This entails outlining obligations and tasks, environment deadlines, and ensuring Absolutely everyone have an understanding of CAPA's importance.
"Action to eliminate the cause of a uncovered nonconformity or other undesirable circumstance," according to the FDA, is what corrective action is. Though preventive action is called "an action to get rid of the reason for a possible nonconformity or other undesirable circumstance," preventive action is the alternative.
The importance of a powerful corrective and preventive action (CAPA) procedure may be connected get more info to two critical factors – consumer satisfaction and regulatory compliance.
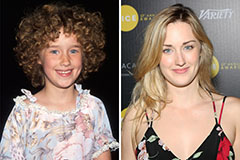 Ashley Johnson Then & Now!
Ashley Johnson Then & Now! Marla Sokoloff Then & Now!
Marla Sokoloff Then & Now! Sam Woods Then & Now!
Sam Woods Then & Now! Rachael Leigh Cook Then & Now!
Rachael Leigh Cook Then & Now! Nicki Minaj Then & Now!
Nicki Minaj Then & Now!